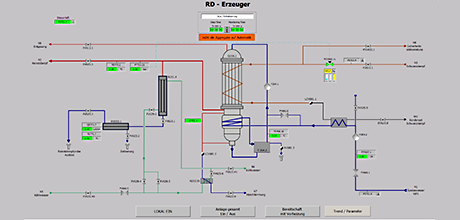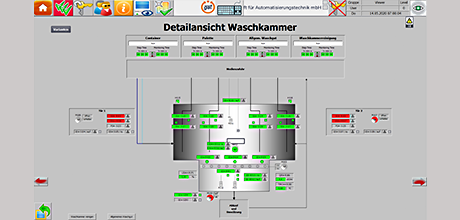
Container washing plant according to GMP & FDA
gat planned and supplied the retrofit for the control and EMSR equipment of an existing container washing plant for the production of infusion solutions. Processing and acceptance were carried out according to GMP and FDA regulations.